Abstract
Introduction:
Auto-immune and Inflammatory Diseases (AID) have been associated with myeloproliferative neoplasms (MPN) in a large population-based study (Kristinsson et al. Haematologica 2010). In myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML), epigenetic regulators TET2 and IDH1/2 were more frequently mutated in patients with AID, suggesting potentially common pathogenesis pathways (Zhao et al. Leukemia 2021). However, in the context of MPN, AID specific features remain poorly characterized, and no study has reported to date the mutational landscape of MPN patients with AID. The objectives of our study were to describe the clinical and molecular characteristics of MPN patients with associated AID and evaluate its impact on patient's outcome.
Methods:
A total of 1541 patients were diagnosed with MPN according to WHO criteria between January 2011 and January 2021 in our center, of whom 998 had a molecular analysis by next generation sequencing (NGS) targeting a panel of 36 genes involved in MPN, performed at diagnosis and/or during follow-up. AID diagnosis was based on international criteria, and all cases have been reviewed by internal medicine experts. Patients with AID induced by interferon-alpha treatment were not included.
Results:
The median age of our whole cohort was 51.3 years IQR[40.4-63.2]. Our cohort included 522 (34%), 709 (46%) and 229 (15%) diagnosis of polycythemia vera (PV), essential thrombocytemia (ET) and primary myelofibrosis (MF) respectively. A total of 100 patients (6.6%) had AID and were compared to the remaining 1441 MPN patients without AID. There were more females (66 (66%) versus 769 (53%), p=0.019) within the AID group compared to non-AID patients. MPN subtype, driver mutation, complete blood counts at diagnosis did not differ between the two groups. Occurrence of thrombosis and hemorrhage episodes did not vary either (44 (44%) versus 564 (39%), p=0.356).
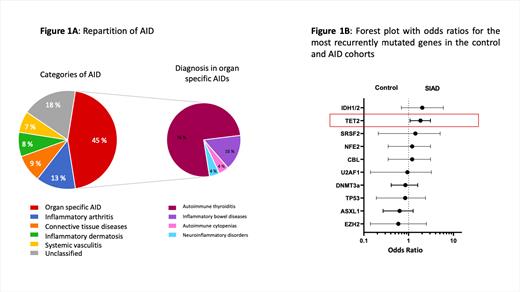
AID diagnosis was prior to MPN in 34% of cases, concomitant in 12% and posterior in 31% of cases. AID diagnosis included 45 (45%) organ-specific AID (mainly autoimmune thyroiditis, n=34), 13 (13%) inflammatory arthritis, 9 (9%) connective tissue diseases, 8 (8%) inflammatory dermatosis, 7 (7%) systemic vasculitis and 18 (18%) unclassified AID (Figure 1A). The AID fulfilled the required classification criteria in 70 (70%) cases, while complete criteria were not reached in 30 (30%) cases.
The median interval of time between MPN diagnosis and NGS was 7.2 years IQR[2.1-13.3] in the whole cohort. Among patients with available molecular analysis, 44 (62%) and 571 (62%) patients had at least one additional non-driver mutated gene in the AID and control groups respectively. Interestingly, TET2 mutations were more frequent in MPN patients with AID (24 (34%) versus 205 (22%), OR=1.84 95%CI[1.08-3.07], p=0.028, Fig 1B). The prevalence of TET2 mutations did not significantly differ between the AID categories. When focusing on IDH1/2 mutations, as they act on the same biological epigenetic pathway as TET2, IDH1/2 mutations were more frequent in the AID cohort although not statistically significant (4 (6%) versus 27 (3%), OR=2.02 95%CI[0.74-5.51], p=0.27). No other mutations including other epigenetic factors, splicing regulators, transcription factors or high molecular risk mutations, were significantly associated with AID. After a median follow up of 8.3 years IQR[3.7-14.3] in the whole cohort, 10 (10%) and 122 (8%) patients died in the AID and control groups respectively. The presence of AID did not impact overall survival (p=0.82), secondary myelofibrosis free (p=0.98) or MDS/AML transformation free (p=0.53) survivals.
Conclusion
Our study reports on a large retrospective clinically and molecularly annotated cohort the prevalence of AID in MPN patients (6.6%). This prevalence did not differ from that of the general population. Interestingly, our data emphasize a high prevalence of TET2 mutations in patients with both AID and MPN, compared to MPN patients without AID. Although other studies are warranted to better define the causal relationship between MPN and AID, our results may suggest a common pathophysiology as it has been proposed in MDS patients, based on shared genetic susceptibilities with mutations in TET2 that could occur within early hematopoietic progenitors and give rise to both the inflammatory phenotype and myeloid malignancy.
DE and LPZ contributed equally to this work.
Fenaux: Celgene/BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; JAZZ: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Ades: Novartis: Honoraria; Takeda: Honoraria; Abbvie: Honoraria; JAZZ: Honoraria; Celgene: Honoraria, Research Funding. Raffoux: PFIZER: Consultancy; CELGENE/BMS: Consultancy; ABBVIE: Consultancy; ASTELLAS: Consultancy. Kiladjian: Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Taiho Oncology, Inc.: Research Funding; PharmaEssentia: Other: Personal fees. Benajiba: Pfizer: Research Funding; Gilead: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal